Compressibility Factor
Compressibility Factor: Overview
This topic covers concepts such as Compressibility of Gas, Compressibility Factor, Variation of Compressibility Factor with Temperature, Variation of Compressibility Factor with Pressure, and Boyle's Temperature.
Important Questions on Compressibility Factor
Van der Waal’s real gas, act as an ideal gas, at which conditions ?
At which one of the following temperature – pressure conditions the deviation of a gas from ideal behaviour is expected to be minimum ?
The isotherms of a gas are shown below :
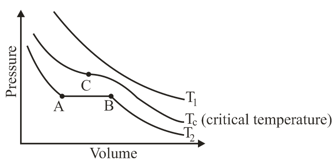
Among the following,
(i) At , the gas cannot be liquefied
(ii) At point , liquid starts to appear at
(ii) is the highest temperature at which the gas can be liquefied
(iv) At point , a small increase in pressure condenses the whole system to a liquid
The correct statements are :
For an ideal gas, the compressibility factor is
For one mole of a van der Waals' gas, the compressibility factor at a fixed volume will certainly decrease, if
[Given : " a " and " b " are standard parameters for van der Waals' gas]
Which of the following represents a plot of compressibility factor vs pressure at room temperature for
In the following compressibility factor v/s pressure graph, which is true?
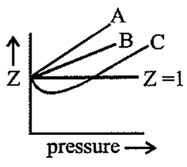
For gas, the compressibility factor, is:
Critical temperature of a gas is ..... Boyle temperature:
At the critical point for gas, value of Then, the value of for the similar conditions of at their respective critical points will be:
Compressibility factor for at and pressure is Find the number of moles of gas required to fill a gas cylinder of capacity under the given conditions.
Which of the following statements regarding compressibility factor , is/are incorrect?
What does Van der Waals gas constant 'a' at higher value indicates?
For one mole of a gas under critical condition, the compressibility factor is-
Correct statement for the graph of real gas is:
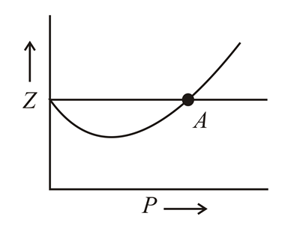
Compressibility factor for any gas is given by the expression: . Considering ideal gas, real gas, and gases at critical state, answer the following questions:
The compressibility of a gas is less than unity at STP, therefore
The temperature at which the real gases obey the ideal gas laws over a wide range of pressure is called
Which of the following represents a plot of compressibility factor (Z) versus P at room temperature for helium?
It is observed that and He gases always show positive deviation from ideal behaviour i.e., Z > 1. This is because
The volume of gas is twice than that of gas The compressibility factor of gas is thrice that of gas at same temperature. The pressures of the gases for equal number of moles are
